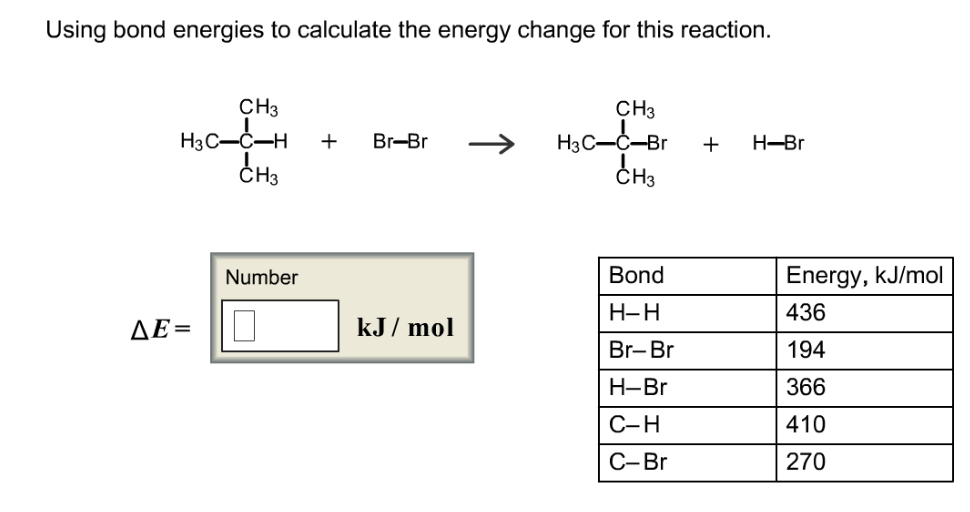
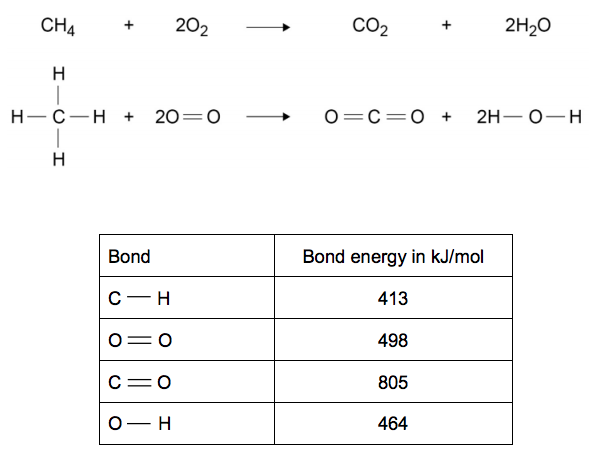
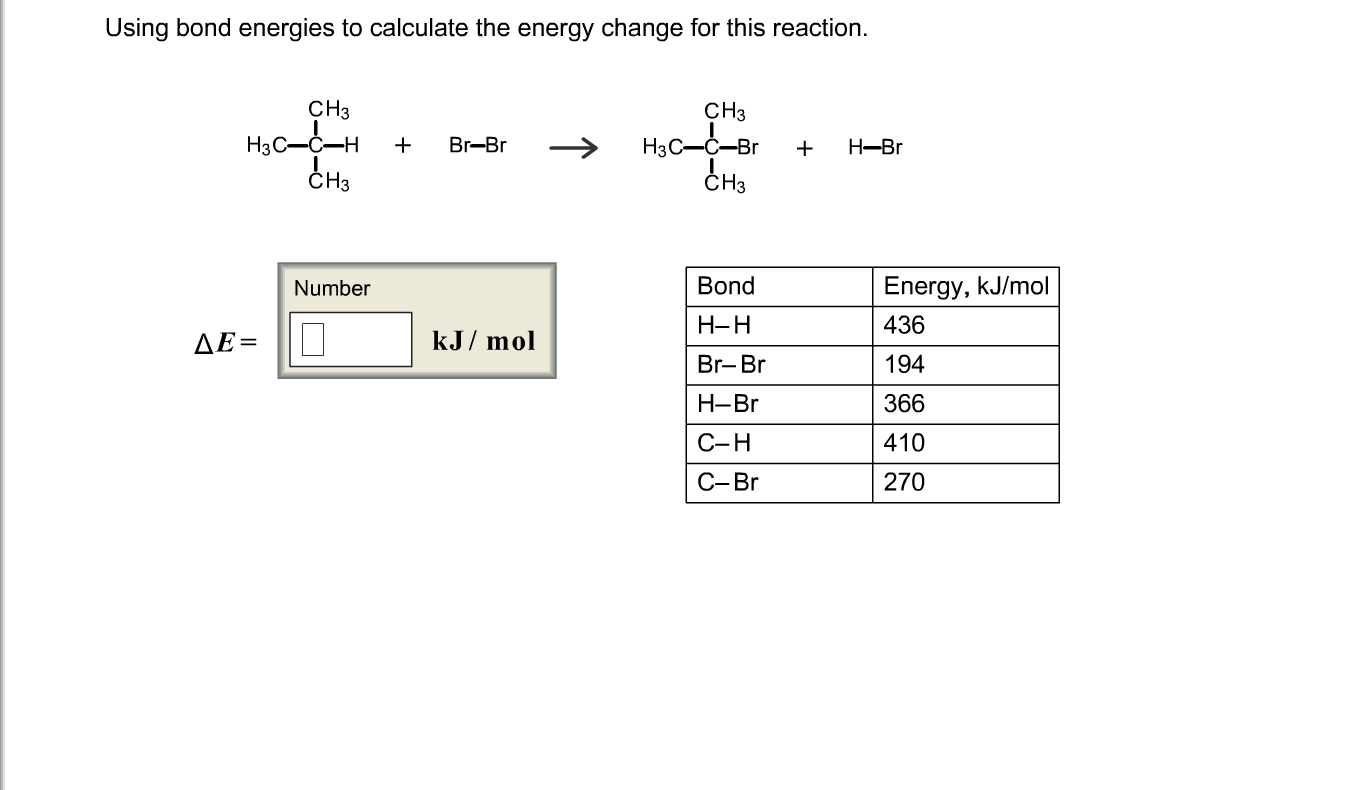
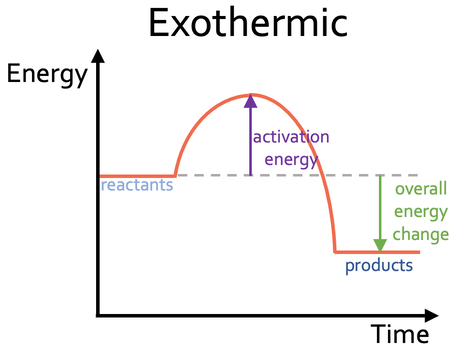
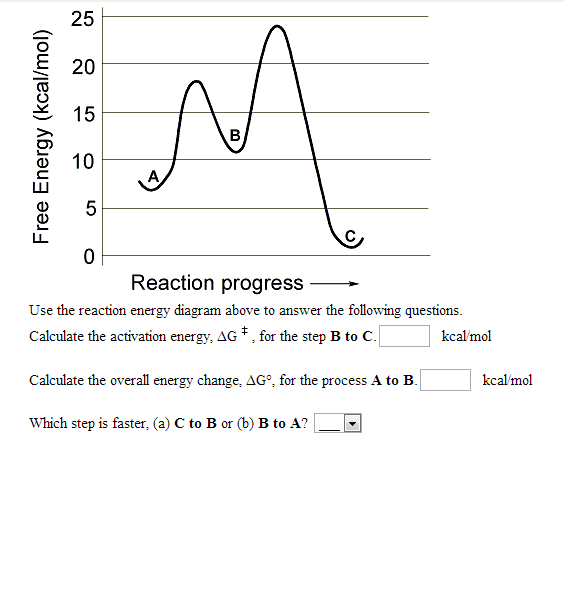
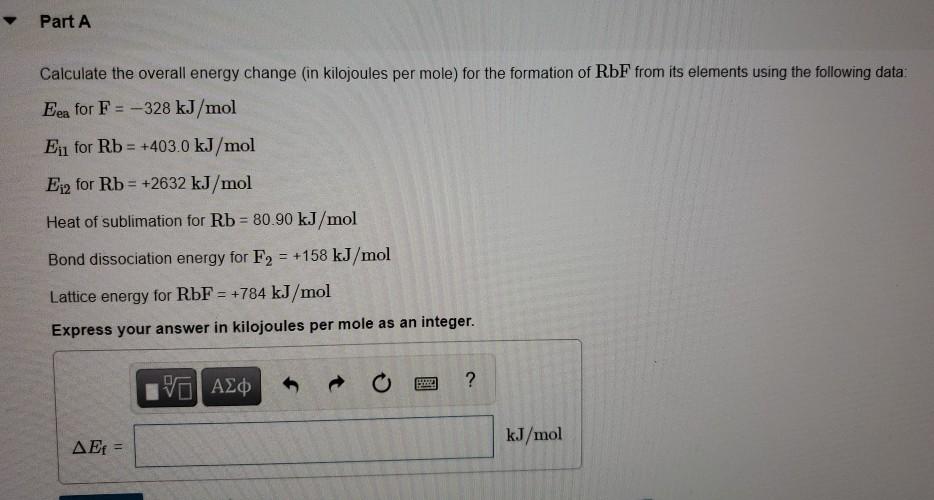
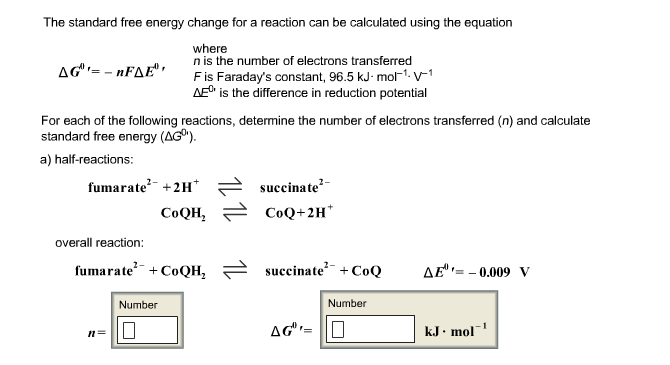
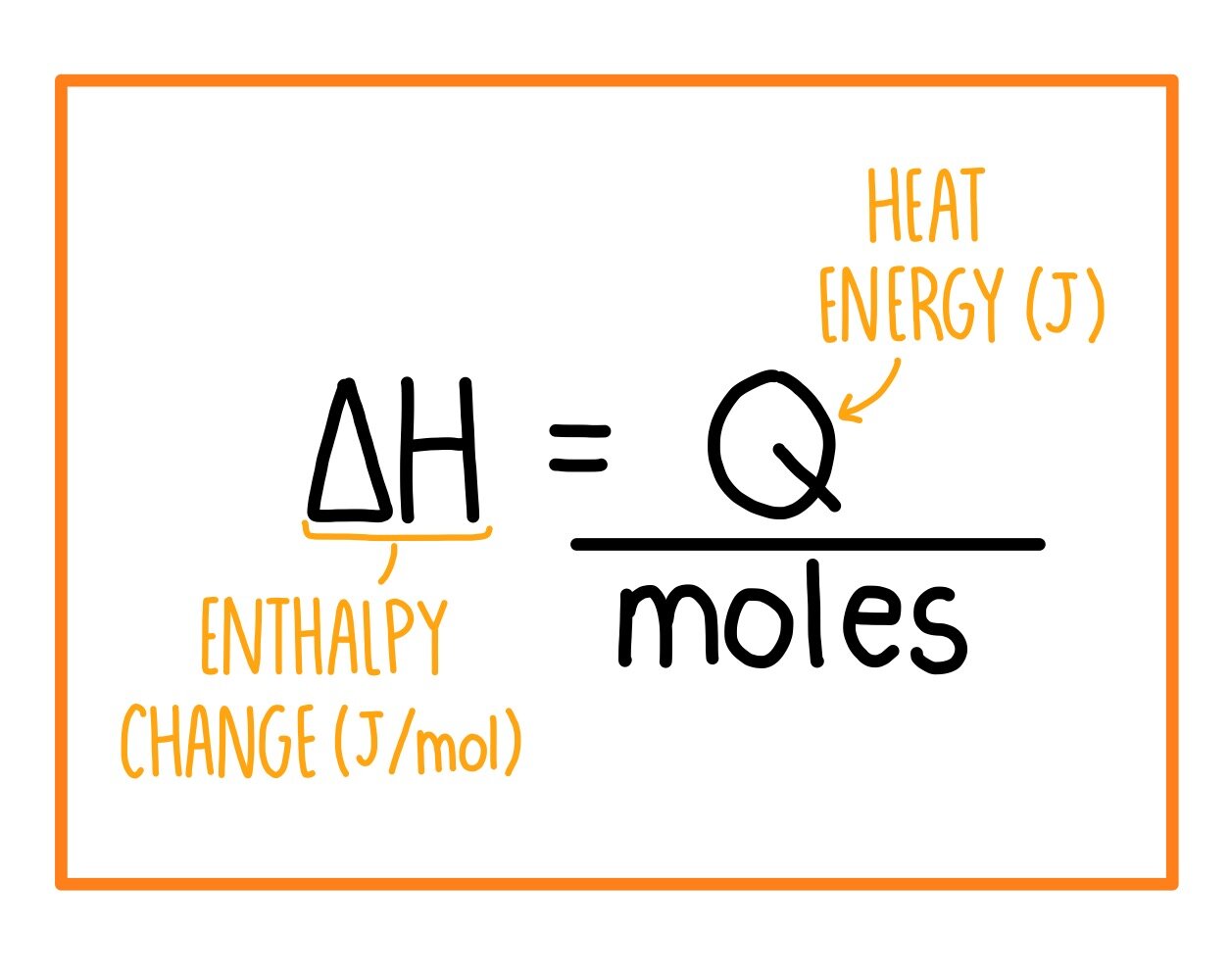Bond energies of H - H and CI - CI are 430 kJ mol^-1 and 242 kJ mol^-1 respectively. Δ Hf for HCl is 91 kJ mol^-1 . What will be the

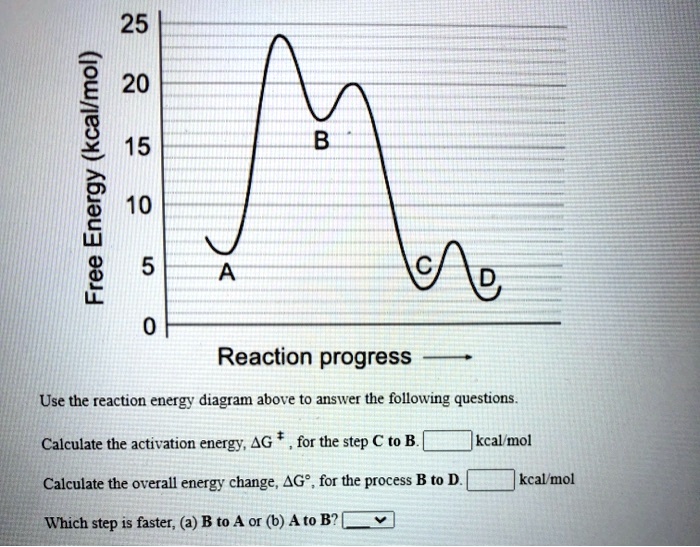
Identifying the Energy Change of Reaction from Reactants to Transition States to Products | Chemistry | Study.com
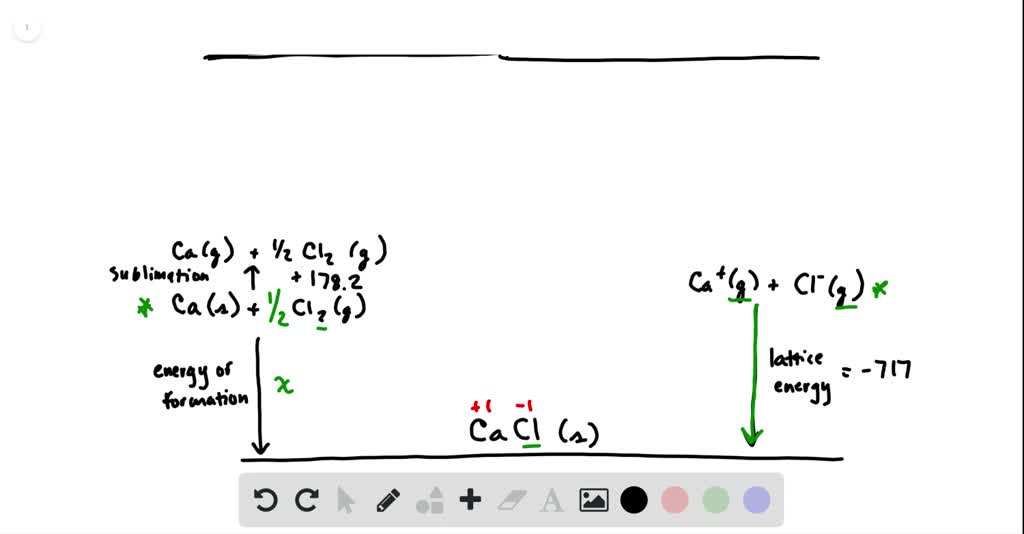
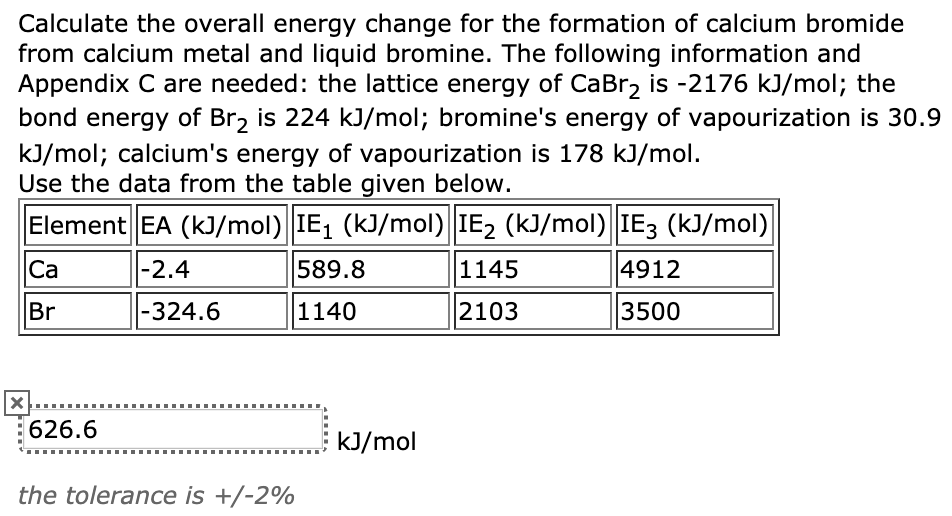
SOLVED:Calculate the overall energy change in kilo joules per mole for the formation of CaCl(s) from the elements. The following data are needed: E ea for Cl(g)=-348.6 kJ / mol Ei 1