SOLVED: Vinegar is a solution of acetic acid, CH3COOH, dissolved in water. A 5.54-g sample of vinegar was titrated to equivalence point by 30.10 mL of 0.100 M Ba(OH)2 . What is

The complete balanced equation for the reaction between barium hydroxide and acetic acid is - brainly.com

sebanyak 50 mL larutan Ba(OH)2 dapat dinetralkan dengan 20 mL larutan CH3COOH 0,1M. tentukan massa - Brainly.co.id

SOLVED: Enter the molecular equation representing aqueous acetic acid neutralized by aqueous barium hydroxide. Express your answer as a balanced molecular equation. Identify all of the phases in your answer: CH3COOH(aq) +

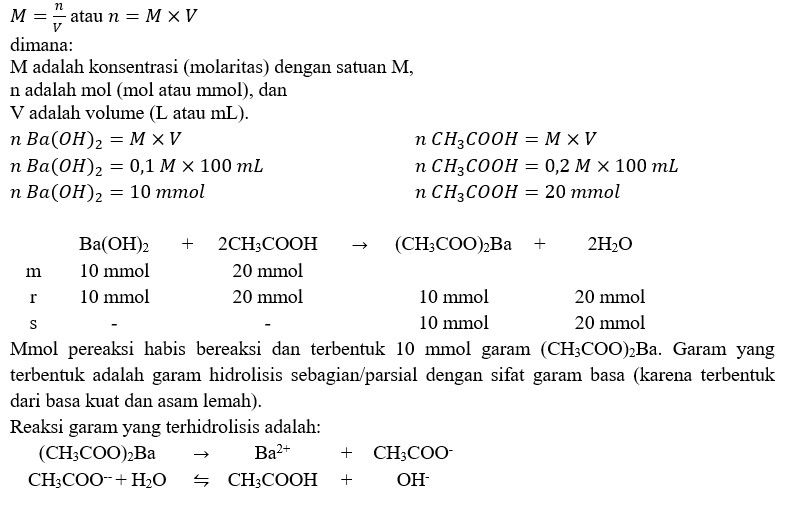
Sebanyak 400ml larutan CH3COOH 0,2M direaksikan dengan 200ml larutan Ba(OH)2 0,1 M. Jika Ka 2•10'-5 . - Brainly.co.id

Write the balanced molecular equation for the reaction between aqueous solutions of acetic acid (CH3COOH) and barium hydroxide, Ba(OH)2. | Homework.Study.com
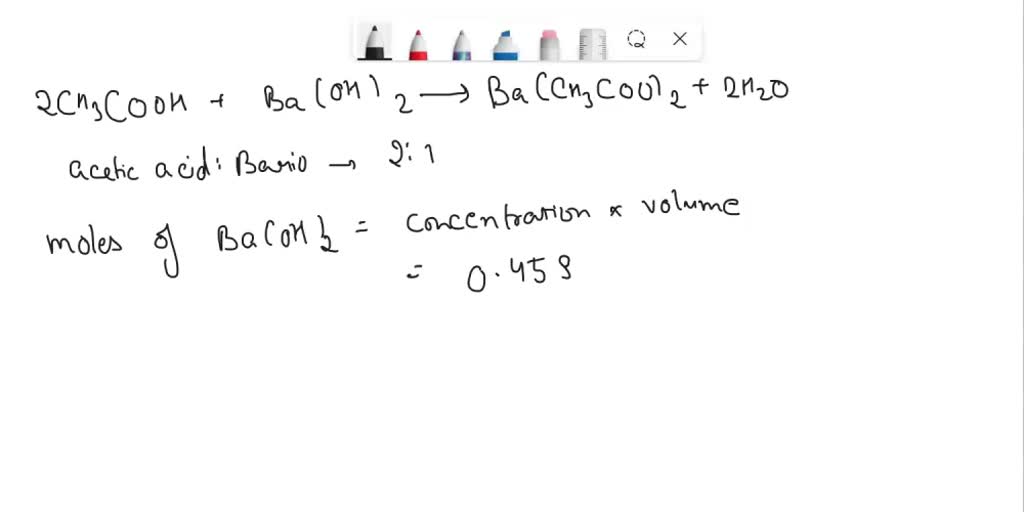
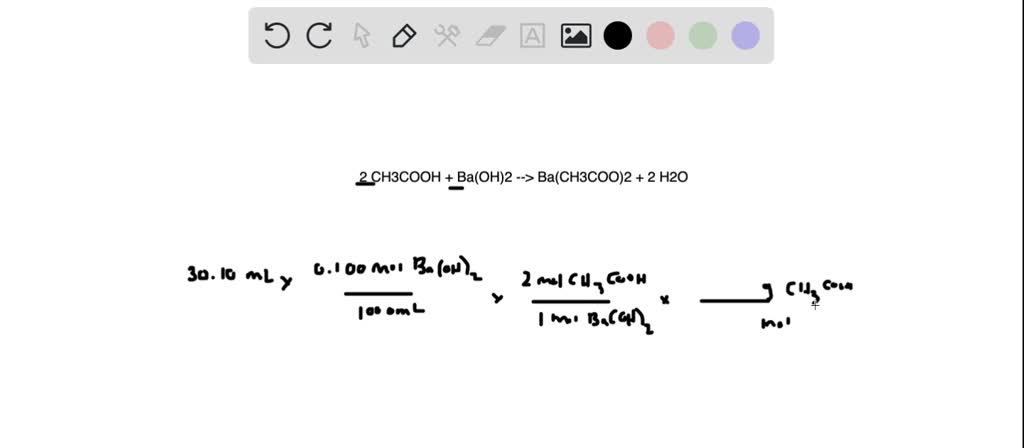
SOLVED: A 25.00 mL sample of vinegar, which is an aqueous solution of acetic acid, CH3COOH, requires 23.15 mL of 0.4587 M barium hydroxide, Ba(OH) 2, to reach the endpoint in a titration.
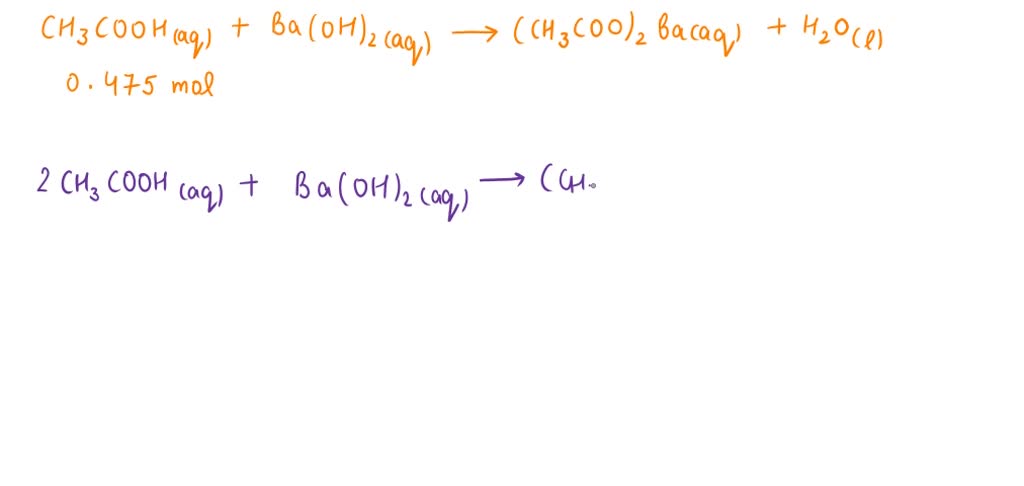
SOLVED: Consider the unbalanced equation for the neutralization of acetic acid: αCH3COOH(aq) + βBa(OH)2(aq) â†' γH2O(l) + δ(CH3COO)2Ba(aq) Determine how many moles of Ba(OH)2 are required to completely neutralize 0.475 mol of

SOLVED: Acetic acid reacts with barium hydroxide. What is the sum of the coefficients for the reactants and products?

SOLVED: Question 10 Not yet answered 100.0 mL of 0.10 M CH3COOH are mixed with 80.0 mL of 0.10 M Ba(OH)2. What determines the pH of the solution after the reaction? Marked

Sebanyak 100 ml Ba(OH)2 0,05m direaksikan dengan 100 ml Ch3cooh 0,1M (Ka Ch3cooh =10'-5) beraoa pH dari - Brainly.co.id